Segment SwitzerlandMARKET ENVIRONMENTBUSINESS PERFORMANCE
The issues healthcare policy focused on in 2021 were managing the Covid crisis and ways to keep costs under control. As the National Council did earlier, the Council of States of the Swiss parliament also rejected the proposed system of reference prices. There are currently two proposals under way in parliament to promote the introduction of electronic prescriptions. And in November, in its report on the Stahl proposal, the Federal Council announced that online sales of OTC medications can be permitted in Switzerland, subject to certain conditions.
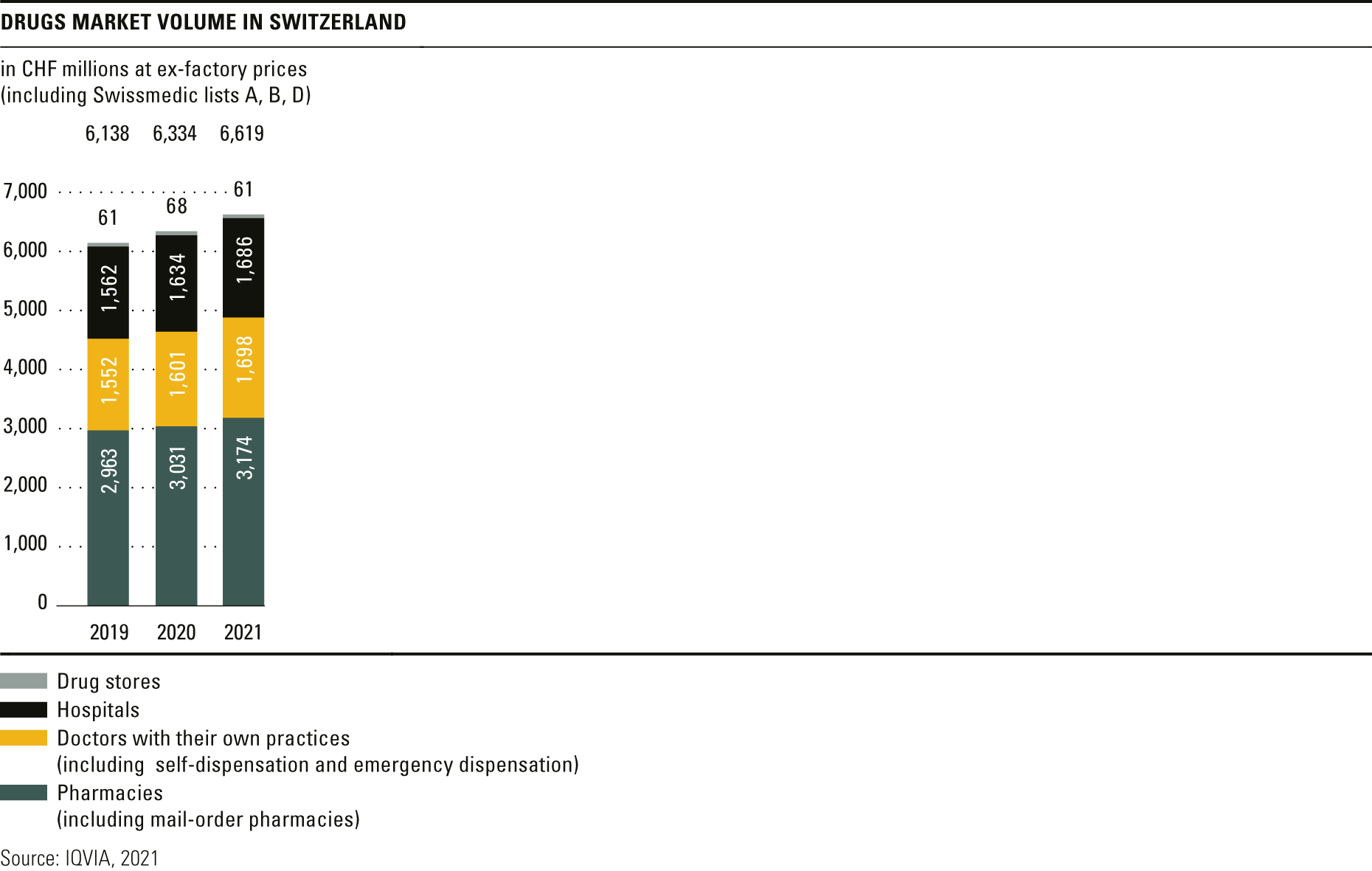
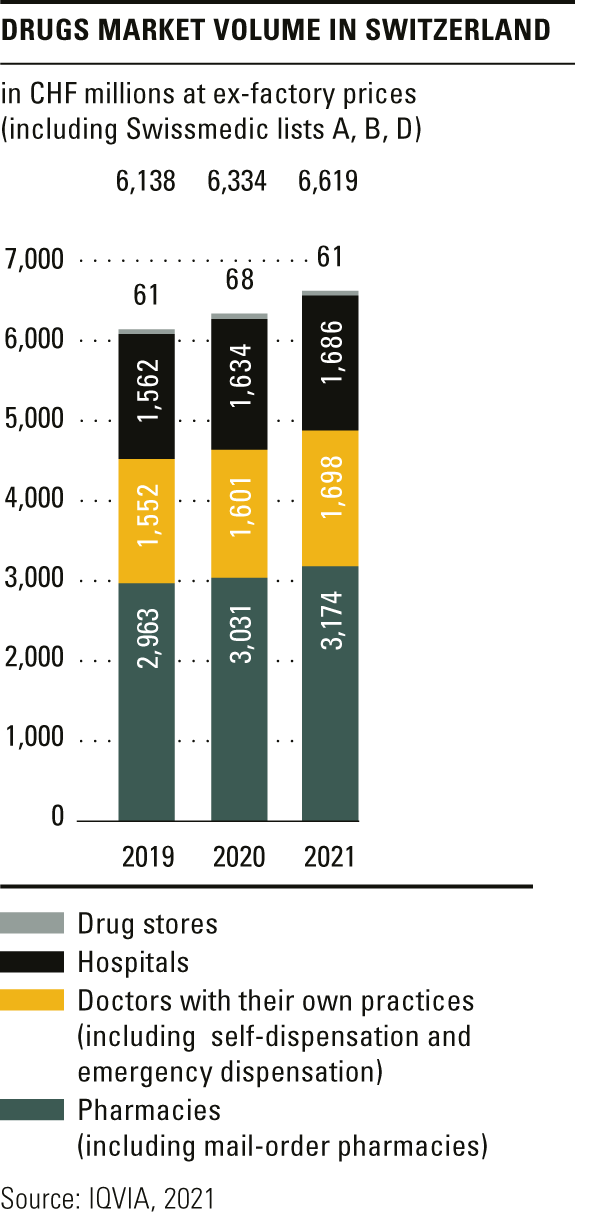
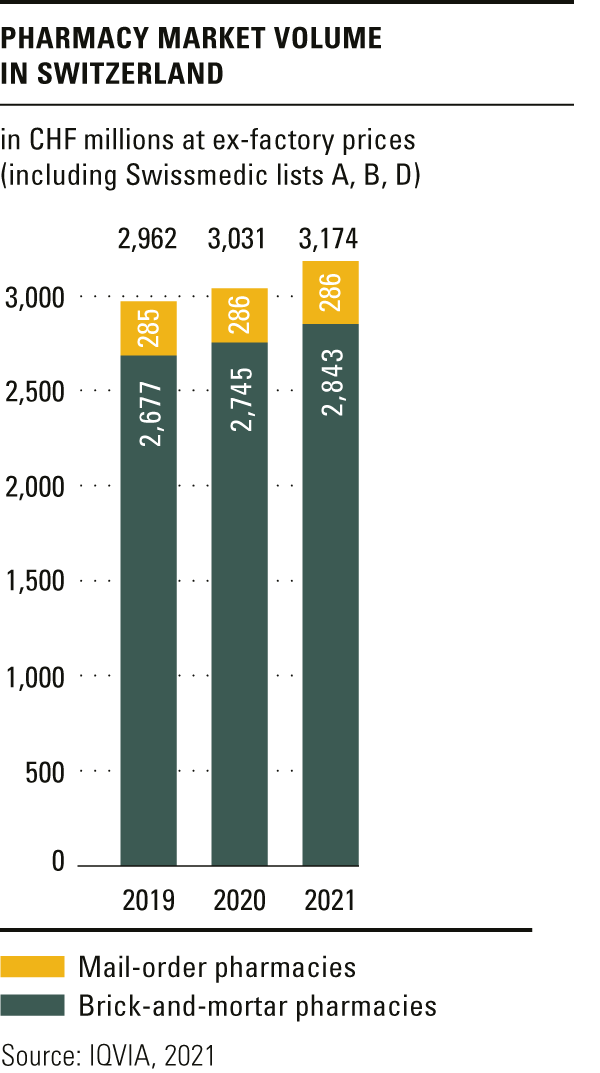
Trends in the market for medicines
The total volume in the drugs market in 2021 amounted to CHF 6.6 billion, a year-on-year rise of over 4 per cent.
Restructuring of the price system for medications
Following the rejection by the National Council during the year under review of the proposal to bring in a reference price system for drugs on which the patent has expired, December saw it fail to pass the Council of States too. Zur Rose reiterated in 2021 that when setting prices for medicines, factors such as ex-factory and generics prices as well as service-based remuneration and distribution margins have to be seen as an inter-related whole. Fair prices and high-quality supply would no longer be possible if the distribution margin were adjusted across the board in isolation. For medium to high-priced medicines in particular, any pricing system that will stand the test of time has to cover costs, otherwise supply to chronically ill people in Switzerland, who frequently depend on drugs in this price segment, will be put at risk.
Pushing rapidly ahead with the introduction of electronic prescriptions
Electronic prescriptions are a key factor when it comes to digitalising healthcare. Both chambers of parliament currently have a motion pending that seeks to introduce electronic prescriptions in Switzerland. Zur Rose welcomes these moves and is committed to the mandatory nationwide introduction of electronic prescriptions, because the benefits for patients and the entire healthcare system are obvious: it increases patient safety by removing error-prone media breaks and cuts out both forged prescriptions and follow-up costs due to incorrect medication.
Liberalisation of online sales of OTC medications
The publication in November of the Federal Council report on the proposal by National Councillor Jürg Stahl triggered movement in the discussion on the liberalisation of online sales of OTC medications. Back in April 2020 the Federal Council had turned down an application by Zur Rose for exceptional permission to sell OTC emergency, cold and flu medications online during the pandemic. In November 2021 it stated in its report that online OTC sales can be permitted subject to certain conditions: “The spread of new technologies, online shopping and the increasing quality and security of IT-supported solutions and software mean it is now possible to consider innovative approaches to ensure quality of care and patient safety with online sales other than a doctor’s prescription. The Covid-19 crisis has considerably speeded up technological development and the use of such processes in the healthcare sector. Digital applications and telemedicine have demonstrably grown in importance.”1 The intention is to hold a consultation on amending the Therapeutic Products Act (TPA) by early 2023.
1 Report of the Federal Council in response to proposal 19.3382 Stahl dated 22 March 2019: online sales of OTC medications, 24 November 2021